Fact-check: Covifor Is Not A COVID-19 Vaccine, Not Slated For Test In Africa As Claimed In This Viral Message
Claim: A viral WhatsApp message has claimed that Covifor injection, manufactured by the Indian pharmaceutical company, Hetero, is a COVID-19 vaccine expected to be tested in Africa.
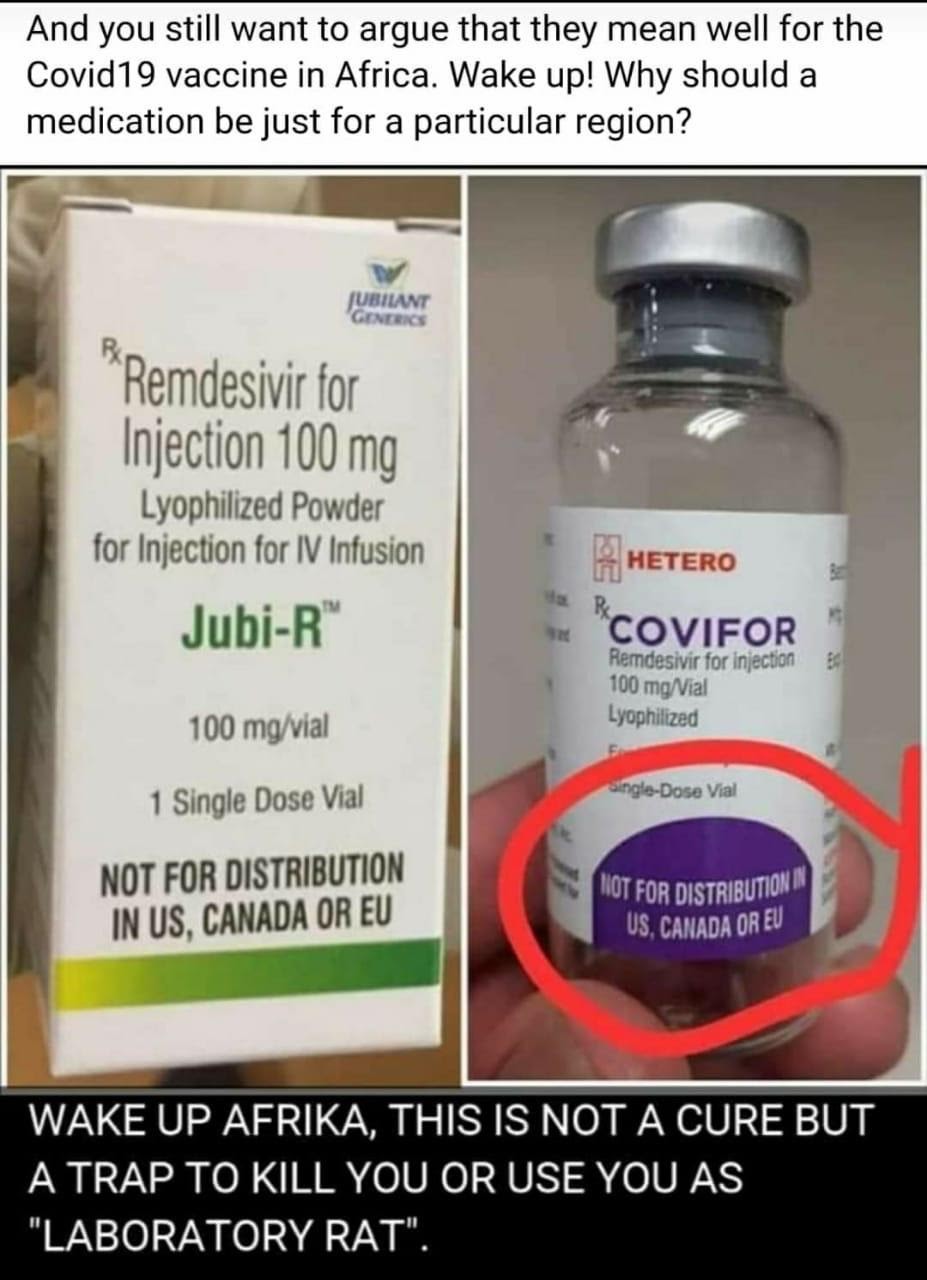
The message attached the picture of the injectable treatment with the label “Not for Distribution in US, Canada or EU” and added a caption that states “Wake up Africa, this is not a cure but a trap to kill you as laboratory trap”.
Verdict: False. A fact-check has revealed that Covifor is not a Covid-19 vaccine. It is a treatment for the Covid-19 patients with severe symptoms of the disease and has been available in 127 countries around the world since June, 2020.
Full Text
A viral WhatsApp message has claimed that Covifor injection, manufactured by the Indian pharmaceutical company, Hetero, is a COVID-19 vaccine expected to be tested in Africa.
The message has been circulating on different social media platforms, majorly WhatsApp since September, 2020.
On Twitter, an account with the name GPAN (@AfricaRepublic) posted the message with the caption “Africa must wake up” on September 2, 2020.
A reply to the tweet by Ssembogo Kwame (@SsembogoJohn3) added that “We’re dying, they want to finish us.”
Another reply by a user with the name blackgirl (@blackgi56617250) said “I tell us this people they think they are very strong but…no.”
These replies suggest that many users believed the post as true.
Verification
Covifor Is Not Covid-19 Vaccine
According to the Hetero, Covifor is an injection for the treatment of Covid-19 patients with severe symptoms of the disease but not a vaccine.
The company, in a statement released on June 24, 2020, said with the Covifor, “we hope to reduce the treatment time of a patient in a hospital, thereby reducing the increasing pressure on the medical infrastructure, overburdened currently due to accelerating covid-19 infection rates.
“We are working closely with the Government and Medical Community to make ‘Covifor’ quickly accessible to both public and private healthcare settings across the country.”
The pharmaceutical company added, “Covifor is the first generic brand of Remdesivir which is indicated for the treatment of COVID19 patients in adults and children, hospitalised with severe symptoms of the disease”.
“The drug is available in 100 mg vial (Injectable). It needs to be administered intravenously in a hospital, critical care setting, under the supervision of a registered medical practitioner.”
Covifor Is Available In Some European Countries
Contrary to the claim that Covifor is produced to be tested in African countries, the injectable treatment is sold in some European countries that include Georgia, Armenia, Belarus, etc.
According to the Gilead agreement, remdesivir (generic name for Covifor) was expected to be sold in low and middle income 127 countries around the world.
“The countries consist of nearly all low-income and lower-middle income countries, as well as several upper-middle- and high-income countries that face significant obstacles to healthcare access.
“The regulatory approval status of remdesivir varies by country, and the distribution of remdesivir within each country listed below is subject to local laws and regulations,” the agreement stated.
The agreement added that “the licenses are royalty-free until the World Health Organization declares the end of the Public Health Emergency of International Concern regarding Covid-19, or until a pharmaceutical product other than remdesivir or a vaccine is approved to treat or prevent Covid-19, whichever is earlier.”
There are many Covid-19 vaccines currently being approved by different countries around the world.
The United Kingdom has approved AstraZaneca produced by Oxford University while Biontech-Pfizer’s vaccine is currently being administered in some European countries.
Conclusion
The claim that Covifor is a vaccine produced to be tested in Africa is false. The injectable treatment was produced by an Indian pharmaceutical company and is available in 127 countries around the world.
This fact-check is produced per HumAngle partnership with the Dubawa 2020 Fellowship to facilitate the ethos of “truth” in journalism and enhance media literacy in Nigeria
Support Our Journalism
There are millions of ordinary people affected by conflict in Africa whose stories are missing in the mainstream media. HumAngle is determined to tell those challenging and under-reported stories, hoping that the people impacted by these conflicts will find the safety and security they deserve.
To ensure that we continue to provide public service coverage, we have a small favour to ask you. We want you to be part of our journalistic endeavour by contributing a token to us.
Your donation will further promote a robust, free, and independent media.
Donate Here